Generic Drug Absorption: How Your Body Takes in Generic Medications
When you take a generic drug absorption, the process by which a generic medication enters your bloodstream to start working. Also known as bioequivalence, it’s the quiet rule that makes generics reliable — not just cheaper, but just as effective as the brand-name version. If your body doesn’t absorb the drug the same way, it won’t treat your condition the same way. That’s why the FDA requires generics to match the brand in how fast and how much of the drug gets into your blood. It’s not about the pill’s color or shape — it’s about the science inside.
What controls this absorption? Three big things: the drug formulation, how the active ingredient is mixed with fillers, coatings, and binders, the pharmacokinetics, how your body moves, breaks down, and gets rid of the drug, and your own biology — stomach acid, food in your gut, even your genetics. A generic pill might look different, but if it’s approved, it delivers the same amount of medicine at the same speed. That’s why millions of people use generics daily without noticing a difference. But when absorption fails — because of bad manufacturing, wrong excipients, or poor testing — that’s when side effects or treatment failure happen.
You’ll find posts here that dig into exactly how this works. Some explain why certain generics work better than others, even when they’re labeled the same. Others show how inactive ingredients — the stuff that’s not the medicine — can actually change how quickly your body absorbs the drug. You’ll see real examples: how a generic version of a blood thinner might behave differently if its coating dissolves too slow, or why a generic antibiotic might need to be taken on an empty stomach when the brand didn’t. There are stories about patients who switched and felt off — and why. And you’ll learn how regulators test this, what the FDA looks for, and how companies try to game the system to delay competition. This isn’t theory. It’s what happens when your pill hits your stomach.
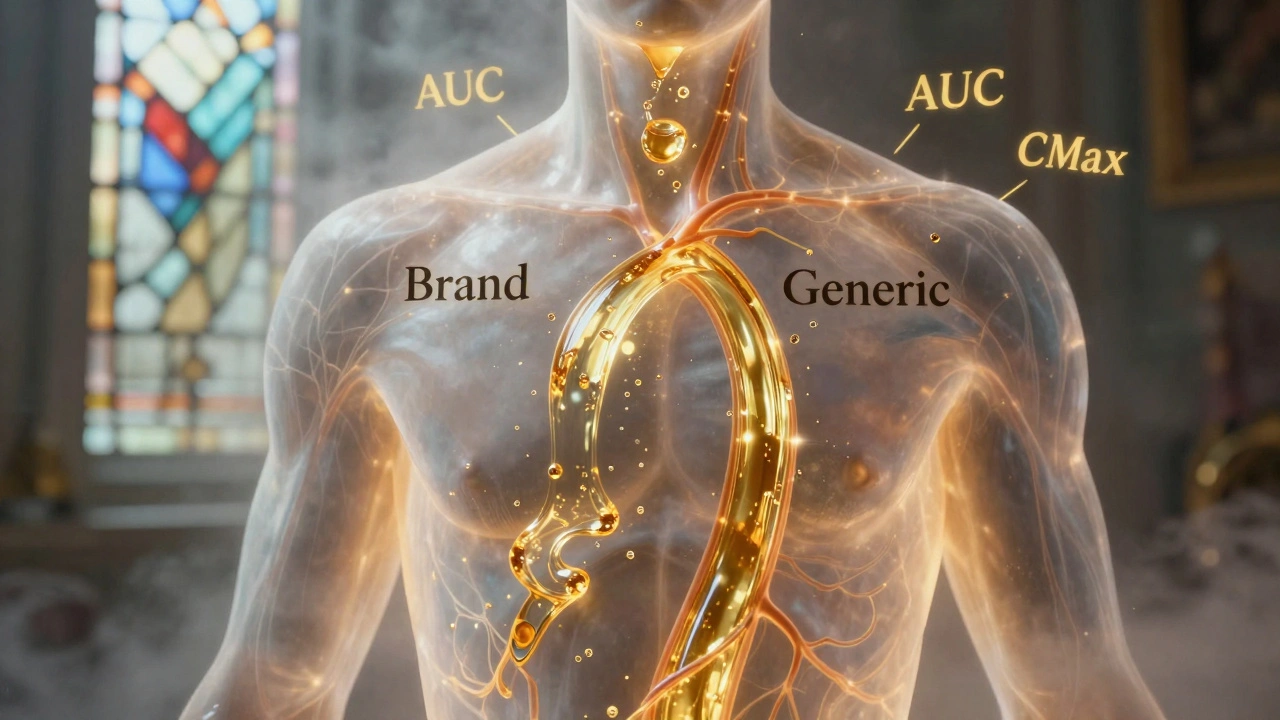
Generic Absorption Rates: How They Must Match Brand Drugs to Be Safe and Effective
Generic drugs must match brand-name absorption rates within strict 80-125% limits to be approved. Science shows they’re nearly identical in effectiveness - with only 3-5% average differences. Learn how bioequivalence works and why generics are safe.