When you pick up a generic pill at the pharmacy, you might wonder: is it really the same as the brand-name version you’ve been taking? The answer isn’t about looks, price, or packaging - it’s about what happens inside your body. Specifically, how fast and how much of the drug gets into your bloodstream. That’s called absorption rate, and for generics to be approved, they must match the brand-name drug within strict limits.
What Does "Bioequivalence" Really Mean?
Bioequivalence isn’t a marketing term. It’s a science-based standard enforced by the FDA and other global regulators. It means a generic drug must deliver the same amount of active ingredient into your blood at the same speed as the brand-name version. This isn’t about being "close enough" - it’s about being clinically interchangeable. The rule is simple: the 90% confidence interval for the ratio of geometric means between the generic and brand drug must fall entirely between 80% and 125%. That’s not a 45% tolerance - it’s a statistical gate. If even one point of that interval slips outside 80-125%, the generic is rejected. Two key measurements are used to prove this:- AUC (Area Under the Curve) - measures total drug exposure over time. Think of it as how much medicine your body absorbs overall.
- Cmax (Maximum Concentration) - measures how quickly the drug reaches its peak level in your blood. This tells you how fast it starts working.
Real-World Data Shows Generics Are Extremely Close
Here’s where most people are surprised: the average difference between generics and brand drugs is tiny. A review of over 2,000 FDA-approved bioequivalence studies found that the mean difference in AUC was just 3.56%. For Cmax, it was 4.35%. That’s less than 5% - not 20%, not 45%. In nearly 98% of cases, the generic differed from the brand by less than 10%. A 2016 analysis of 2,070 studies showed that the geometric mean ratios were almost exactly 1.00 for both AUC and Cmax, with a standard deviation of only ±0.04 to ±0.06. In plain terms: generics are almost identical in how they behave in the body. You might hear stories about someone feeling different on a generic. But those cases are rare - and often not about absorption at all. The FDA has tracked over 14,000 generic approvals since 2008. Only 12 cases raised potential safety concerns - a failure rate of 0.08%. That’s less than one in a thousand.Why Do Some Generics Look Different or Feel Different?
Generics can’t look like the brand-name drug. U.S. trademark laws force differences in color, shape, size, and even flavor. That’s why your generic pill might be blue instead of white, or oval instead of round. These changes are purely cosmetic. They don’t affect how the drug works. Sometimes, patients report side effects or reduced effectiveness after switching. But when studies control for placebo effects and patient expectations, the differences vanish. A 2023 meta-analysis of 47 studies with over 9,600 patients found no significant difference in outcomes between generic and brand-name cardiovascular drugs. The same holds true for antidepressants, blood pressure meds, and most other common prescriptions. There are exceptions - and they’re important. For drugs with a narrow therapeutic index (NTI), like warfarin, digoxin, or phenytoin, even a small change in blood levels can cause harm. For these, the FDA requires tighter limits: 90-111% for AUC. Pharmacists are trained to flag these cases, and many states require prescriber approval before switching.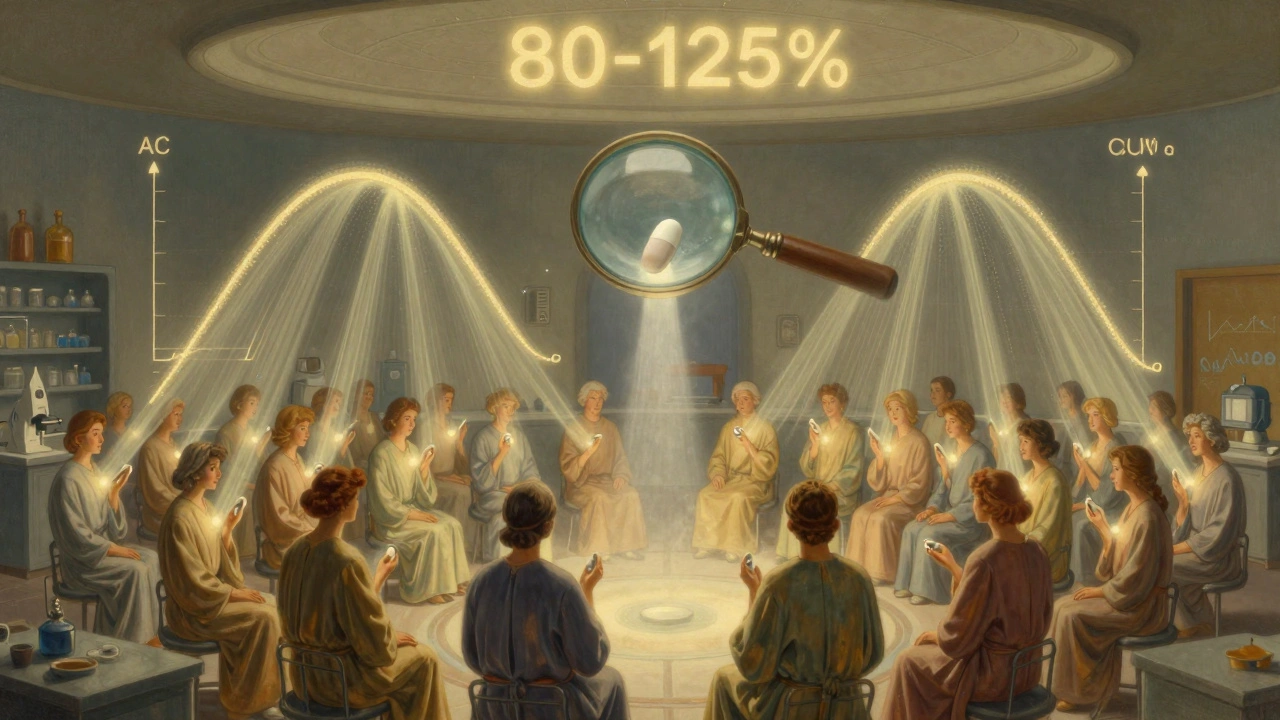
Dissolution Isn’t the Same as Absorption
You might read that some generics dissolve slower or faster in lab tests. That’s true. One study found that 54% of generic products showed different dissolution rates compared to their brand-name counterparts. For example, a generic version of nifedipine dissolved much slower, while another of amoxicillin dissolved faster. But here’s the key point: dissolution ≠ absorption. A pill can dissolve quickly in a test tube but still be absorbed slowly in the gut. Or it can dissolve slowly but still release the drug at the right pace in the body. That’s why the FDA doesn’t rely on dissolution tests alone. They require real human data - in vivo studies - to prove bioequivalence. If a generic passes the 80-125% bioequivalence test, it doesn’t matter if its dissolution profile looks different. The body absorbs it the same way. That’s why the FDA continues to approve generics even when their dissolution curves don’t match the brand.What About the "B-Rated" Generics?
The FDA’s Orange Book rates generics with an "A" or "B" code. "A" means therapeutically equivalent. "B" means there’s a documented bioequivalence concern - usually because the generic doesn’t meet the 80-125% standard in all cases, or because it’s a complex formulation like a topical cream or extended-release tablet. If you’re switching from a brand to a generic, your doctor or pharmacist should check the Orange Book rating. For most drugs, an "A" rating means you can switch without worry. But for NTI drugs, or if you’ve been stable on a brand for years, some experts recommend sticking with what you know - even if the generic is technically equivalent. That’s not because generics are unsafe. It’s because consistency matters in chronic conditions. If you’re on a stable dose of levothyroxine and feel fine, switching to a different generic manufacturer might introduce a tiny, unpredictable change - even if it’s within legal limits.Global Standards Are Mostly the Same
The U.S. isn’t alone. The European Medicines Agency (EMA) uses the exact same 80-125% range. Japan’s PMDA is stricter for some drugs, requiring 85-115%. But the core principle is universal: if the drug doesn’t behave the same in the human body, it doesn’t get approved. This global alignment means generics made in India, China, or Germany can be sold in the U.S. - and vice versa - because they all follow the same science.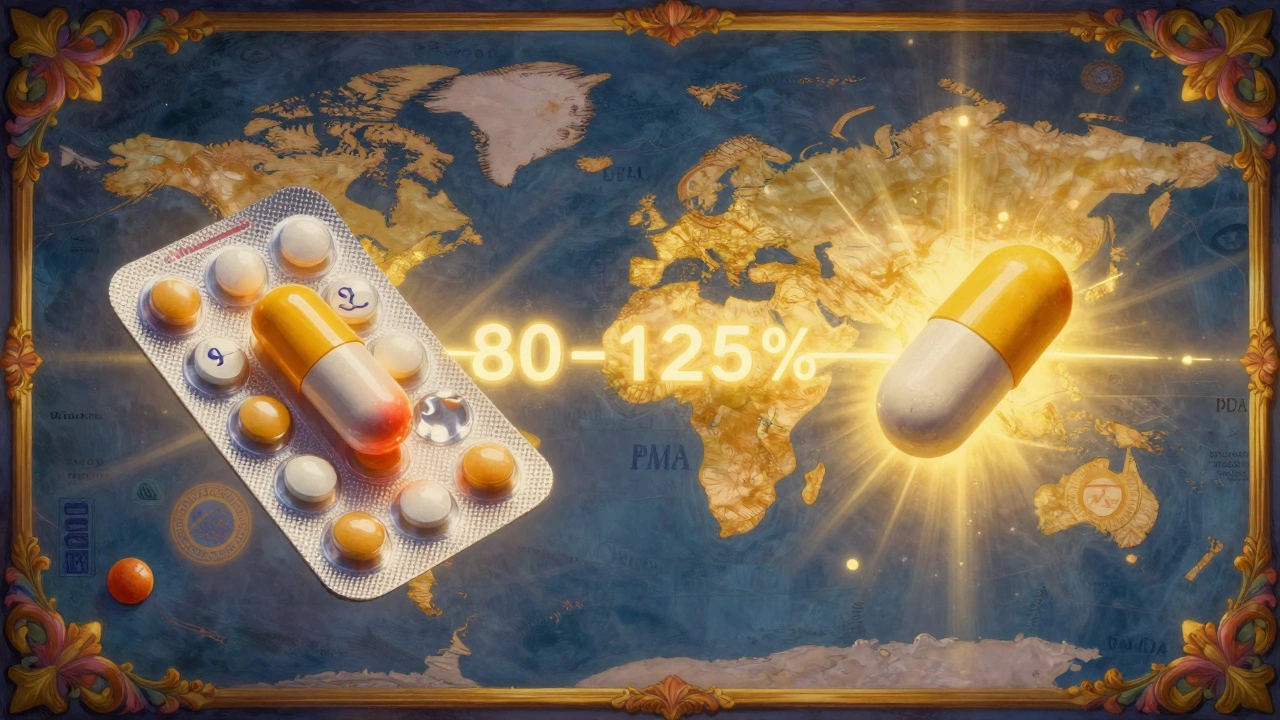
Why This Matters for You
Generics save the U.S. healthcare system over $300 billion a year. They make up 90% of all prescriptions but cost only 23% of total drug spending. That’s not just good for your wallet - it’s good for public health. Without generics, millions of people couldn’t afford their meds. You don’t need to fear generics. You need to understand them. If your doctor prescribes a generic, it’s because it’s been proven to work the same way. If you’re switching from brand to generic, monitor how you feel for a few weeks. But don’t assume a difference means the drug is failing. Most often, it’s your brain - not your body - that’s reacting. If you’re on a narrow therapeutic index drug, talk to your pharmacist. Ask if the generic you’re getting has an "A" rating. If you’ve had issues before, ask about staying with the same manufacturer. Consistency helps.What’s Changing Now?
The FDA is moving toward using computer modeling to predict bioequivalence, especially for complex drugs like inhalers or topical creams. This could cut down on the need for human studies in the future. But the 80-125% rule isn’t going anywhere. It’s been tested for decades. It works. The next big challenge is biosimilars - generic versions of biologic drugs like insulin or cancer treatments. These are harder to copy because they’re made from living cells, not chemicals. But even here, the FDA is applying the same logic: prove it behaves the same in people. The standard is still 80-125% for pharmacokinetics, with extra requirements for safety.Bottom Line
Generic drugs must match brand-name drugs in absorption - not just in name. The science is clear: the differences are tiny, the standards are tight, and the safety record is excellent. If your generic looks different, tastes different, or costs less - that’s fine. As long as it’s approved, it’s not a compromise. It’s a proven alternative. Don’t let myths scare you away from generics. They’re not second-rate. They’re science-backed, cost-effective, and just as effective as the brand - when they’re made right.Are generic drugs really as effective as brand-name drugs?
Yes. Generic drugs must meet the same strict bioequivalence standards as brand-name drugs. They must deliver the same amount of active ingredient into the bloodstream at the same rate. Studies show the average difference in absorption is less than 5%, and over 98% of generics differ by less than 10%. The FDA approves only those generics that perform the same in real human studies.
Can I switch between different generic brands safely?
For most drugs, yes. All approved generics must meet the same 80-125% bioequivalence standard. But if you’re on a narrow therapeutic index drug like warfarin or levothyroxine, switching between manufacturers can cause small, unpredictable changes. If you’re stable on one generic, it’s often safer to stick with it. Talk to your pharmacist about consistency.
Why do some people say generics don’t work as well?
Most reports of differences are based on perception, not science. Changes in pill color, size, or taste can trigger psychological reactions - sometimes called the "nocebo effect." Studies show that when patients don’t know which drug they’re taking, they report no difference in effectiveness. Real clinical outcomes are nearly identical between generics and brands.
What’s the difference between dissolution and absorption?
Dissolution is how fast a pill breaks down in a lab test. Absorption is how much of the drug actually enters your bloodstream. A generic might dissolve faster or slower in a test tube, but still be absorbed the same way in your body. That’s why the FDA requires human studies - not just lab tests - to approve generics.
Are generics regulated differently in other countries?
Most major regulators, including the FDA and EMA, use the same 80-125% bioequivalence standard. Japan requires tighter limits (85-115%) for some drugs. But globally, the science is aligned: if the drug doesn’t behave the same in the human body, it’s not approved.
Should I avoid generics if I have a chronic condition?
Not necessarily. For most chronic conditions, generics are safe and effective. But for drugs with a narrow therapeutic index - like thyroid meds, seizure drugs, or blood thinners - consistency matters. If you’re stable on a brand or a specific generic, talk to your doctor before switching. Don’t assume you need to change - but don’t fear switching either, if your provider recommends it.
Ada Maklagina
December 5, 2025 AT 15:45Been taking generics for years. Never had an issue. My blood pressure med? Same as the brand. My thyroid? Same. I don’t get the drama.
They’re cheaper. They work. Move on.
Harry Nguyen
December 6, 2025 AT 18:28Of course the FDA says they’re fine. They’re paid off by Big Pharma to keep the generic industry quiet. You think they want you saving $300 billion? Nah. They want you hooked on $500 pills with fancy packaging.
And don’t even get me started on how India and China make these. You know what’s in those pills? Dust and hope.
Chris Brown
December 7, 2025 AT 04:04It is imperative to recognize that the regulatory framework governing bioequivalence is not merely a statistical convenience-it is a foundational pillar of public health policy. The 80-125% confidence interval is not arbitrary; it is the product of decades of clinical pharmacokinetic research and peer-reviewed validation.
Those who dismiss generics based on anecdotal experience fail to appreciate the rigorous, evidence-based methodology underpinning their approval. The burden of proof lies with the skeptic, not the regulator.
Stephanie Fiero
December 8, 2025 AT 08:27Y’all need to chill. I switched from brand Zoloft to generic last year and I swear I felt worse at first. Turned out I was just freaking out because the pill was blue instead of white. Once I stopped obsessing, I was fine.
Don’t let your brain sabotage your meds. Your body doesn’t care what color the pill is.
Also-pharmacists are your friends. Ask them questions. They know this stuff.
Laura Saye
December 10, 2025 AT 01:27The real question isn’t whether generics are bioequivalent-it’s whether we’ve created a healthcare system where the only metric of value is cost reduction.
Yes, the data says they’re equivalent. But when we reduce human physiology to AUC and Cmax curves, we forget that healing isn’t just pharmacokinetics. It’s trust. It’s consistency. It’s the quiet reassurance that your body knows what it’s getting.
Maybe the 2% of people who report differences aren’t wrong-they’re just more sensitive to the symbolic weight of change.
Michael Dioso
December 12, 2025 AT 01:21LMAO so now we’re supposed to trust a 98% success rate? That’s like saying 98% of your Tinder dates are chill-still, two of them are gonna ghost you or show up with a pet iguana.
And don’t even get me started on the dissolution thing. One generic dissolves like a sugar cube, another like a rock. But hey, if your blood levels are ‘within range,’ it’s all good?
My grandma’s on warfarin. I wouldn’t let her switch generics unless I personally vetted the batch. Call me paranoid. I call it common sense.
Mark Curry
December 13, 2025 AT 03:27I used to be scared of generics too. Then I started checking the Orange Book. If it’s an A rating, I trust it. If it’s a B, I ask my pharmacist why.
Most of the time, it’s just a weird formulation-like a delayed-release version that’s not technically the same. But for 95% of pills? Totally fine.
Also, the price difference is insane. I saved $80/month on my statin. That’s a Netflix subscription and a pizza. Worth it.
luke newton
December 13, 2025 AT 23:46You people are naive. The FDA doesn’t test every single batch. They test a few from a factory in Mumbai and say ‘good enough.’
What happens when the factory runs out of the right filler? What if the lab technician is tired? What if the heat in the warehouse messes with the stability?
You think your life is worth $0.50 a pill? Think again.
Ali Bradshaw
December 15, 2025 AT 14:08My dad’s on digoxin. Switched from brand to generic last year. No issues. His cardiologist said it’s fine. He’s 78. Still walks 5 miles a day.
Don’t fear the generic. Fear the fear.
And if you’re really worried? Stick with the same manufacturer. That’s the real trick-not avoiding generics, but staying consistent.
an mo
December 15, 2025 AT 22:08Let’s be brutally honest: the 80-125% range is a regulatory compromise, not a scientific ideal. It’s the bare minimum to keep generics on the market without triggering lawsuits from brand manufacturers.
And yes, 98% of generics are fine-but that 2%? That’s the one that kills someone. And then the FDA says ‘it’s within limits.’
That’s not safety. That’s statistical gambling with human lives.