FDA Bioequivalence: What It Means for Generic Drugs and Your Health
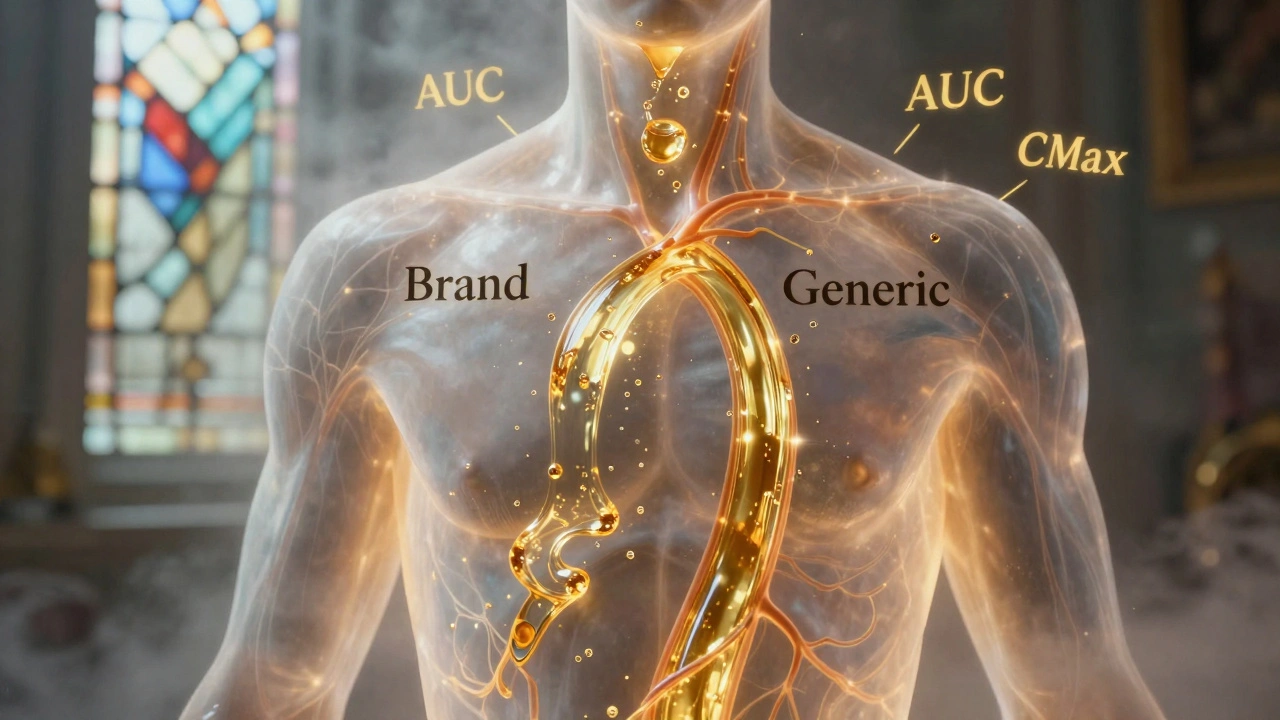
When you pick up a generic pill, you want to know it does the same job as the brand-name version. That’s where FDA bioequivalence, a regulatory standard that proves a generic drug performs the same way in the body as its brand-name counterpart. Also known as drug equivalence, it’s the invisible gatekeeper that lets cheaper versions reach you safely. The FDA doesn’t just accept claims—it demands proof. Every generic must show it releases the same amount of active ingredient at the same rate as the original. That’s not guesswork. It’s done through strict clinical tests, usually with healthy volunteers who take both versions and have their blood drawn over time to track absorption.
This process ties directly to ANDA approval, the application pathway generic manufacturers use to get FDA clearance without repeating expensive clinical trials. The ANDA doesn’t ask if the drug cures a disease—that’s already proven by the brand. It asks: does it get into your bloodstream the same way? If yes, it’s approved. If not, it’s rejected. And this isn’t rare. Over 90% of prescriptions filled in the U.S. are generics, and nearly all of them passed bioequivalence testing. But not every drug makes it through. Some complex formulations—like extended-release pills or inhalers—can’t be easily copied, which is why you still see brand-only options even after patents expire.
Bioequivalence also connects to generic drugs, medications that contain the same active ingredient as brand-name drugs but cost up to 85% less. It’s not about cutting corners—it’s about matching performance. A generic that fails bioequivalence could mean your blood pressure doesn’t drop, your seizure control slips, or your blood thinner doesn’t work right. That’s why the FDA requires tests to be within 80–125% of the brand’s results. That narrow range ensures safety, even for drugs with tight therapeutic windows like warfarin or thyroid meds.
You might hear people say generics aren’t as good. But if it carries the FDA’s approval stamp, that’s not true. The real issue? Some manufacturers cut costs in ways that don’t affect bioequivalence but might cause other problems—like inconsistent pill coatings or inactive ingredients that trigger allergies. That’s why you’ll find posts here on inactive ingredients, compounding errors, and how to read your medication guide. The system works, but it’s not perfect. And that’s why understanding bioequivalence matters: it’s your first line of defense against fake or poorly made generics.
Below, you’ll find real stories and data on how generics are tested, why some drugs still have no generic alternatives, how long FDA reviews take, and what happens when bioequivalence gets challenged in court. Whether you’re saving money on prescriptions or just want to know your pills are safe, these posts give you the facts—not the hype.
Generic Absorption Rates: How They Must Match Brand Drugs to Be Safe and Effective
Generic drugs must match brand-name absorption rates within strict 80-125% limits to be approved. Science shows they’re nearly identical in effectiveness - with only 3-5% average differences. Learn how bioequivalence works and why generics are safe.