Bioequivalence Standards: What Makes Generic Drugs Truly Equal
When you pick up a generic pill, you expect it to do the same job as the brand-name version—and bioequivalence standards, the scientific benchmarks that prove two drugs deliver the same effect in the body. Also known as drug equivalence criteria, these standards are the invisible gatekeepers that decide whether a generic drug can legally replace a brand-name one. Without them, you’d be guessing if your cheaper pill would work—or if it might even hurt you.
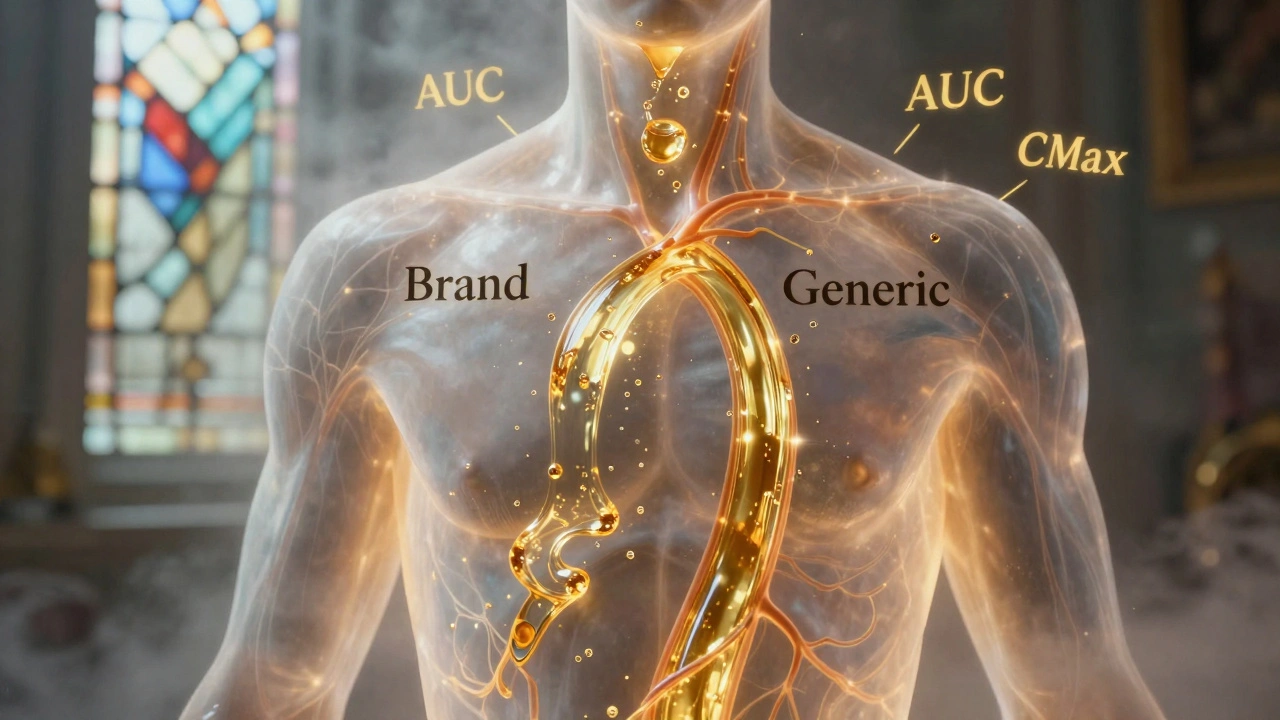
These standards aren’t just paperwork. They’re based on real data from human studies that measure how fast and how much of the drug gets into your bloodstream. This is called pharmacokinetics, how your body absorbs, distributes, metabolizes, and gets rid of a drug. For a generic to pass, its blood levels must match the brand-name drug within strict limits—usually 80% to 125% of the original. That’s not a guess. It’s a lab-tested rule enforced by the FDA, the U.S. agency that reviews every generic drug application before it hits shelves. If the numbers don’t line up, the drug gets rejected—even if it looks identical, costs less, or has the same active ingredient.
But here’s the catch: bioequivalence doesn’t mean identical. It means functionally the same. Two drugs can have different fillers, colors, or shapes and still pass. That’s why some people notice differences in side effects or how quickly they feel relief—it’s not the active ingredient, it’s the inactive ones. That’s where things like drug formulation, the full recipe of active and inactive components that determine how a pill behaves in your body come into play. A poorly designed formulation can mess with absorption, even if the active ingredient passes the bioequivalence test.
And it’s not just about getting the dose right. The timing matters too. If a drug is absorbed too fast, you might get a spike in side effects. Too slow, and it won’t work when you need it. That’s why bioequivalence studies don’t just measure total exposure—they track how the levels rise and fall over time. This is why some generics work perfectly for one person but not another. Your metabolism, what you ate before taking it, even your gut health can shift how the drug behaves—so the standard has to be tight enough to protect everyone.
What you’ll find in the posts below is a clear picture of how this system works in real life. From how the FDA reviews generic applications to why some drugs never get generics at all, these stories show you the hidden rules behind your medicine cabinet. You’ll see how patents, manufacturing quirks, and even legal tricks can delay or block generic entry—even after the patent expires. And you’ll learn how to tell if your generic is truly doing what it should, or if you might need to ask for a different version. This isn’t theory. It’s what’s in your pills—and what you deserve to know.
Generic Absorption Rates: How They Must Match Brand Drugs to Be Safe and Effective
Generic drugs must match brand-name absorption rates within strict 80-125% limits to be approved. Science shows they’re nearly identical in effectiveness - with only 3-5% average differences. Learn how bioequivalence works and why generics are safe.